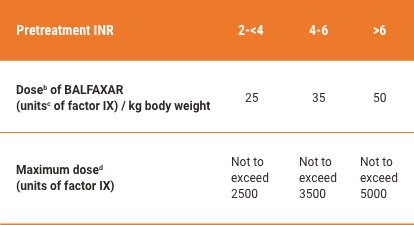
BALFAXAR Dose Is Individualized Based on Patient’s Pretreatment INR and Body Weight1,a
BALFAXAR Dosage for Reversal of VKA Anticoagulation
BALFAXAR1
-
Should not be administered to patients with sensitivity to heparin
-
Is to be administered concurrently with vitamin K
Download the BALFAXAR Dosing Guide
aThe safety and effectiveness of repeat dosing have not been established, and it is not recommended.
bDosing is based on body weight. Dose based on actual potency is stated on the vial, which will vary from 20-32 factor IX units/mL after reconstitution. The actual potency for a 500-unit vial ranges from 400-640 units/vial. The actual potency for a 1000-unit vial ranges from 800-1280 units/vial.
cUnits refer to International Units.
dDose is based on body weight up to but not exceeding 100 kg. For patients weighing more than 100 kg, maximum dose should not be exceeded.
Reference: 1. BALFAXAR, Prothrombin Complex Concentrate (Human) Full Prescribing Information. Paramus, NJ: Octapharma USA, Inc.
Important Safety InformationView More +
WARNING: ARTERIAL AND VENOUS THROMBOEMBOLIC COMPLICATIONS
Patients being treated with Vitamin K antagonists (VKA) therapy have underlying disease states that predispose them to thromboembolic events. Potential benefits of reversing VKA should be weighed against the potential risks of thromboembolic events, especially in patients with the history of a thromboembolic
Important Safety InformationView Less —
WARNING: ARTERIAL AND VENOUS THROMBOEMBOLIC COMPLICATIONS
Patients being treated with Vitamin K antagonists (VKA) therapy have underlying disease states that predispose them to thromboembolic events. Potential benefits of reversing VKA should be weighed against the potential risks of thromboembolic events, especially in patients with the history of a thromboembolic event. Resumption of anticoagulation should be carefully considered as soon as the risk of thromboembolic events outweighs the risk of acute bleeding. Both fatal and non-fatal arterial and venous thromboembolic complications have been reported with BALFAXAR in clinical trials and post marketing surveillance. Monitor patients receiving BALFAXAR for signs and symptoms of thromboembolic events. BALFAXAR may not be suitable in patients with thromboembolic events in the prior 3 months.
BALFAXAR is contraindicated in patients with known anaphylactic or severe systemic reactions to BALFAXAR or any of its components. BALFAXAR is also contraindicated in patients with a known allergy to heparin, a history of heparin-induced thrombocytopenia (HIT), and IgA deficient patients with known antibodies against IgA.
In clinical trials, the most frequent (≥3%) adverse reactions observed in subjects receiving BALFAXAR were procedural pain, wound complications, asthenia, anemia, dysuria, procedural vomiting, and catheter-site-related reaction.
BALFAXAR is derived from human plasma. The risk of transmission of infectious agents, including viruses and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent and its variant (vCJD), cannot be completely eliminated.
Indications
BALFAXAR (prothrombin complex concentrate, human-lans) is a blood coagulation factor replacement product indicated for the urgent reversal of acquired coagulation factor deficiency induced by Vitamin K antagonist (VKA, e.g., warfarin) therapy in adult patients with need for an urgent surgery/invasive procedure.
Please click here for Full Prescribing Information, including BOXED WARNING.
To report suspected adverse reactions, contact Octapharma USA, Inc. at 1-866-766-4860 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.