Confidence at Every Moment
BALFAXAR. Total Support.
Welcome to the Octapharma Support and Resource Site
Customized for HealthTrust Members
BALFAXAR demonstrated similar efficacy and safety to Kcentra in a head-to-head clinical trial1,2
BALFAXAR is stable at room temperature for 8 hours after reconstitution, 2x longer than Kcentra1,3,a
Based on the results of a usability study, the nextaro® transfer device is preferred over the Mix2Vial by HCPs4,b
HealthTrust Members' BALFAXAR Resources
HealthTrust Members Letter

Download our letter to HealthTrust members with information about our initial package
Demonstration Kit

Request a Demonstration Kit
Support backed by Octapharma’s premium customer service platform can help you navigate every step, from ordering to dosing and administration questions.
Dedicated HealthTrust Member Support Center Number
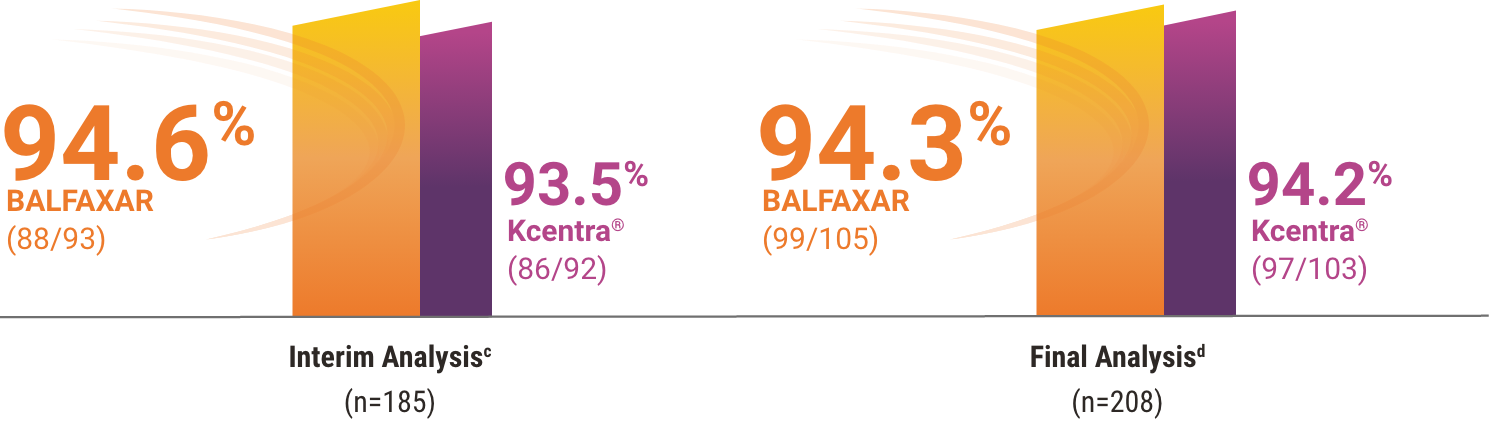
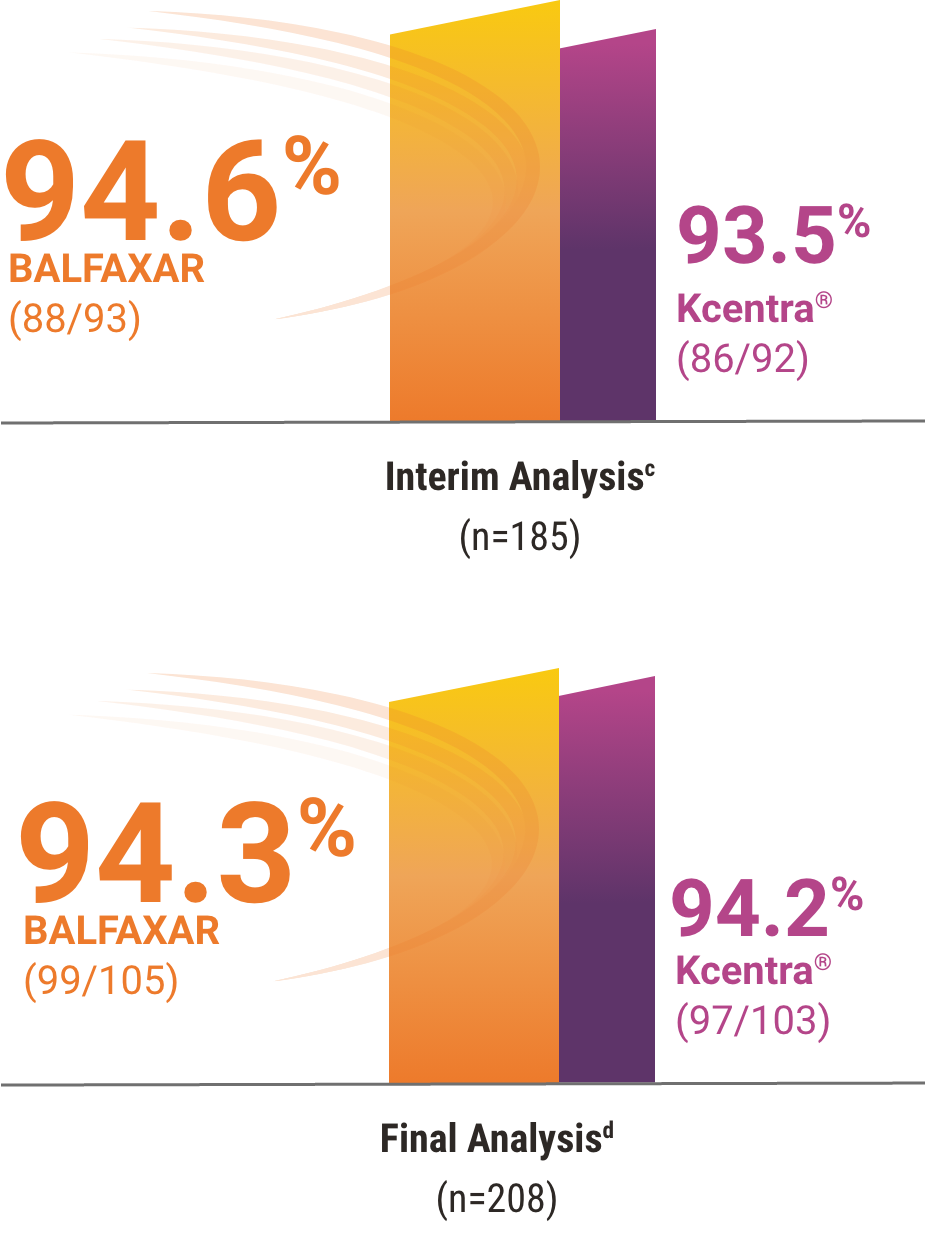
BALFAXAR Was Proven Non-Inferior to Kcentra in Hemostatic
Efficacy in a Head-to-Head Warfarin Reversal Study1
BALFAXAR was found to be clinically non-inferior to Kcentra, resulting in early study termination at the prespecified interim analysis.1, 2
Percentage of patients who achieved effective hemostasis1,2
The median dose of BALFAXAR or Kcentra was 25 IU of factor IX/kg body weight in both treatment groups, ranging from 16 IU/kg to 50 IU/kg in the BALFAXAR group and 15 IU/kg to 50 IU/kg in the Kcentra group.1
The median duration of the infusion was 12 minutes in the BALFAXAR group (ranging from 8 to 50 minutes) and 13 minutes (ranging from 7 to 30 minutes) in the Kcentra group.1
BALFAXAR Resulted in Rapid and Sustained Reduction in INR
78.1% of patients receiving BALFAXAR vs 71.8% in the Kcentra group had a reduction in INR to ≤1.5 at 30 minutes post-infusion in a randomized, controlled trial1,e
Patients on BALFAXAR had sustained INR reduction to <1.3 for up to 24 hours after infusion1
The most common adverse reactions observed (≥3%) in BALFAXAR patients were procedural pain (47.6%), wound complications (14.3%), asthenia (12.4%), anemia (5.7%), dysuria (4.8%), procedural vomiting (3.8%), and catheter-site—related reaction (3.8%).1,f
BALFAXAR Has Convenient Reconstitution and Prolonged Stability1,4,5
Reconstituted BALFAXAR solution can be stored for up to 8 hours at room temperature (20°C to 25°C; 68°F to 77°F), provided sterility of the stored product is maintained.1,a
Based on the results of a usability study, the nextaro® transfer device is preferred over the Mix2Vial by HCPs.4,b
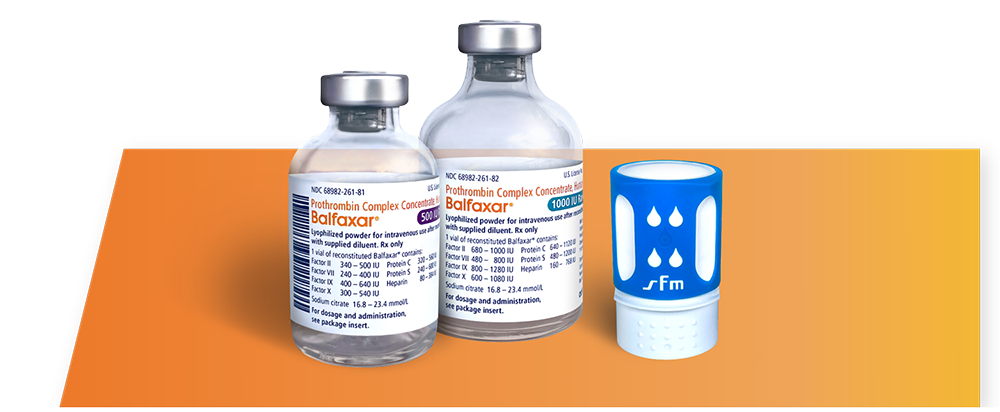
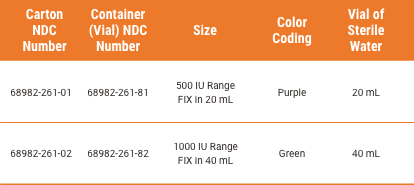
BALFAXAR Is Supplied in Single-Dose Vials and Is Available in 2 Vial Sizes1
To order BALFAXAR, use the associated NDC Number1:
Each carton of BALFAXAR vials contains a nextaro® transfer device and a vial of sterile water for injection.
Components used in the packaging of BALFAXAR are not made with natural rubber latex.
Kcentra is a registered trademark of CSL Behring LLC.
Mix2Vial is a registered trademark of West Pharma. Services IL, Ltd., a subsidiary of West Pharmaceutical Services, Inc.
nextaro® is a registered trademark of sfm medical devices GmbH.
aBALFAXAR can be stored for up to 36 months at 2ºC to 25ºC (36ºF to 77ºF) from the date of manufacture.
bUser preference was determined from the responses of 16 healthcare providers using an 11-item questionnaire about the usability of the nextaro® and Mix2Vial transfer devices.4
cMet prespecified non-inferiority criterion (margin used -15% and P<0.001, statistically significant at prespecified alpha level of 0.01). Hemostatic efficacy at the end of surgery was assessed by the blinded Independent Endpoint Adjudication Board using objective criteria.
dDescriptive analysis of all subjects in the study, including an overrun of subjects during the decision making at the interim analysis.
e78.1% in the BALFAXAR group (n=105) vs 71.8% in the Kcentra group (n=103; proportion difference of 6.3%; 95% CI: -5.5%, 18.0%).1
fThe safety and efficacy of repeat dosing of BALFAXAR have not been established.
References: 1. BALFAXAR, Prothrombin Complex Concentrate (Human) Full Prescribing Information. Paramus, NJ: Octapharma USA Inc. 2. Sarode R, Goldstein JN, Simonian G, Milling TJ Jr. A phase 3, prospective, randomized, double-blind, multicenter, non-inferiority study comparing two four-factor prothrombin complex concentrates for reversal of vitamin K antagonist-induced anticoagulation in patients needing urgent surgery with significant bleeding risk. Blood. 2022;140(Suppl 1):352-353. doi:10.1182/blood-2022-168890 3. Kcentra, Prothrombin Complex Concentrate (Human) Full Prescribing Information. King of Prussia, PA: CSL Behring LLC. 4. Data on File, Octapharma 2023. 5. US Food and Drug Administration. 510K Summary: K183187. March 2019. Accessed September 14, 2023. https://www.accessdata.fda.gov/cdrh_docs/pdf18/K183187.pdf
BALF-0153-POT
Important Safety InformationView More +
WARNING: ARTERIAL AND VENOUS THROMBOEMBOLIC COMPLICATIONS
Patients being treated with Vitamin K antagonists (VKA) therapy have underlying disease states that predispose them to thromboembolic events. Potential benefits of reversing VKA should be weighed against the potential risks of thromboembolic events, especially in patients with the history of a thromboembolic
Important Safety Information
WARNING: ARTERIAL AND VENOUS THROMBOEMBOLIC COMPLICATIONS
Patients being treated with Vitamin K antagonists (VKA) therapy have underlying disease states that predispose them to thromboembolic events. Potential benefits of reversing VKA should be weighed against the potential risks of thromboembolic events, especially in patients with the history of a thromboembolic event. Resumption of anticoagulation should be carefully considered as soon as the risk of thromboembolic events outweighs the risk of acute bleeding. Both fatal and non-fatal arterial and venous thromboembolic complications have been reported with BALFAXAR in clinical trials and post marketing surveillance. Monitor patients receiving BALFAXAR for signs and symptoms of thromboembolic events. BALFAXAR may not be suitable in patients with thromboembolic events in the prior 3 months.
BALFAXAR is contraindicated in patients with known anaphylactic or severe systemic reactions to BALFAXAR or any of its components. BALFAXAR is also contraindicated in patients with a known allergy to heparin, a history of heparin-induced thrombocytopenia (HIT), and IgA deficient patients with known antibodies against IgA.
In clinical trials, the most frequent (≥3%) adverse reactions observed in subjects receiving BALFAXAR were procedural pain, wound complications, asthenia, anemia, dysuria, procedural vomiting, and catheter-site-related reaction.
BALFAXAR is derived from human plasma. The risk of transmission of infectious agents, including viruses and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent and its variant (vCJD), cannot be completely eliminated.
Indications
BALFAXAR (prothrombin complex concentrate, human-lans) is a blood coagulation factor replacement product indicated for the urgent reversal of acquired coagulation factor deficiency induced by Vitamin K antagonist (VKA, e.g., warfarin) therapy in adult patients with need for an urgent surgery/invasive procedure.
Please click here for Full Prescribing Information, including BOXED WARNING.
To report suspected adverse reactions, contact Octapharma USA, Inc. at 1-866-766-4860 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.