Welcome to the Octapharma support
and resource site customized for
HealthTrust members
Dedicated HealthTrust Member Support Center Number
1-833-382-7684
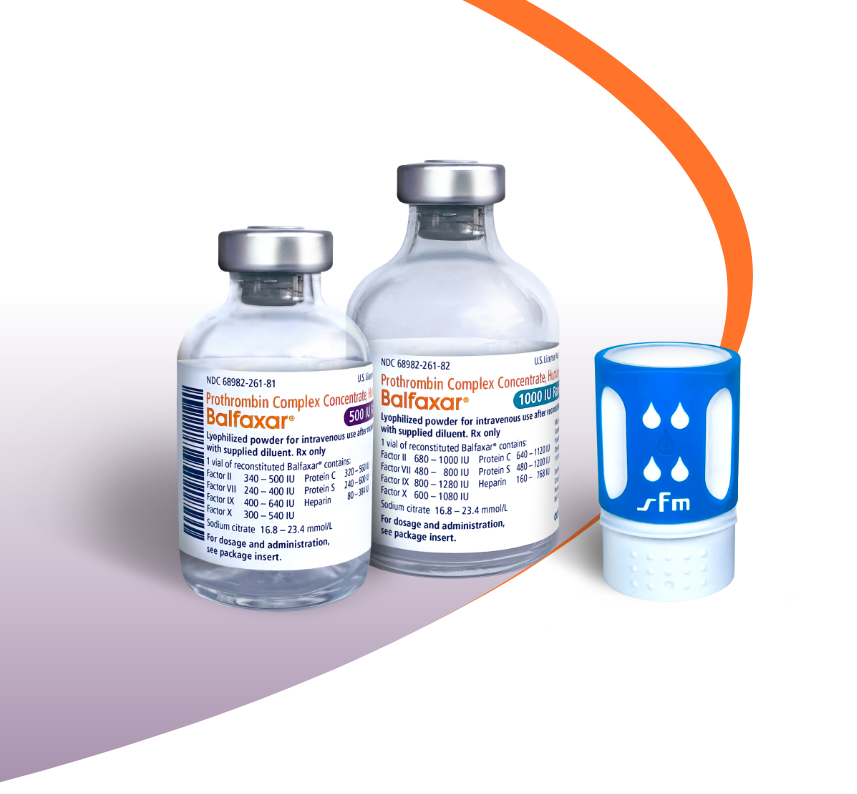
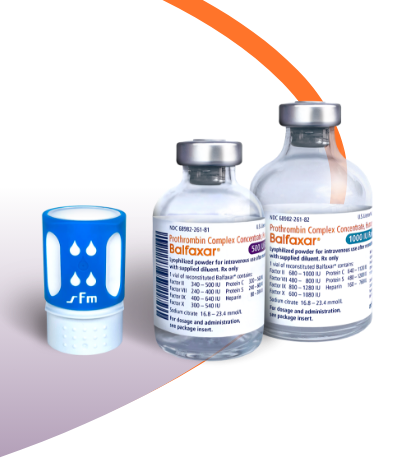
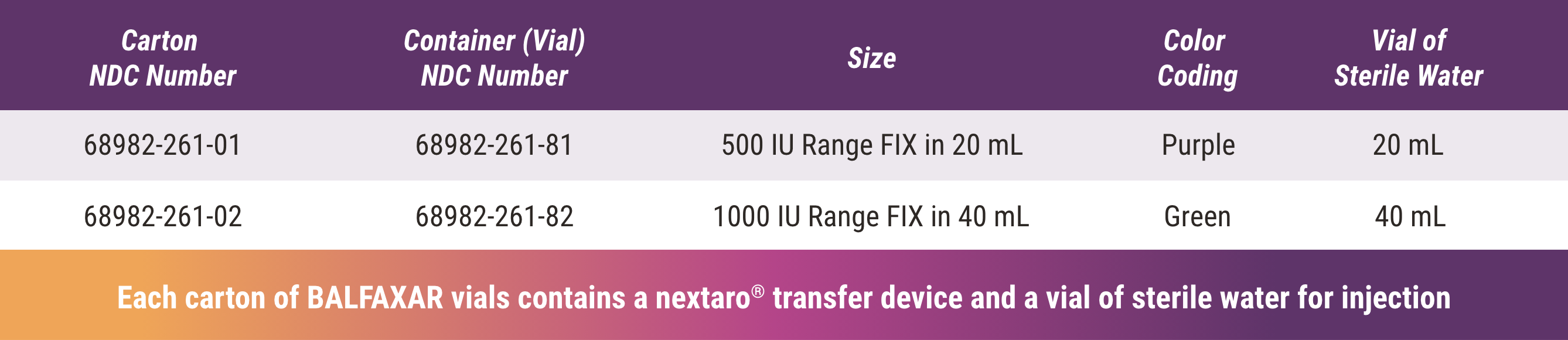
BALFAXAR Is Supplied in Single-Dose Vials and Is Available in 2 Vial Sizes1

The median dose of BALFAXAR or Kcentra® was 25 IU of factor IX/kg body weight in both treatment groups, ranging from 16 IU/kg to 50 IU/kg in the BALFAXAR group and 15 IU/kg to 50 IU/kg in the Kcentra® group.1
The median duration of the infusion was 12 minutes in the BALFAXAR group (ranging from 8 to 50 minutes) and 13 minutes (ranging from 7 to 30 minutes) in the Kcentra® group.1

INR reduction to <1.3 for up to
Kcentra® is a registered trademark of CSL Behring LLC.
Mix2Vial is a registered trademark of West Pharma. Services IL, Ltd., a subsidiary of West Pharmaceutical Services, Inc.
nextaro® is a registered trademark of sfm medical devices GmbH.
aUser preference was determined from the responses of 16 healthcare providers using an 11-item questionnaire about the usability of the nextaro® and Mix2Vial transfer devices.2
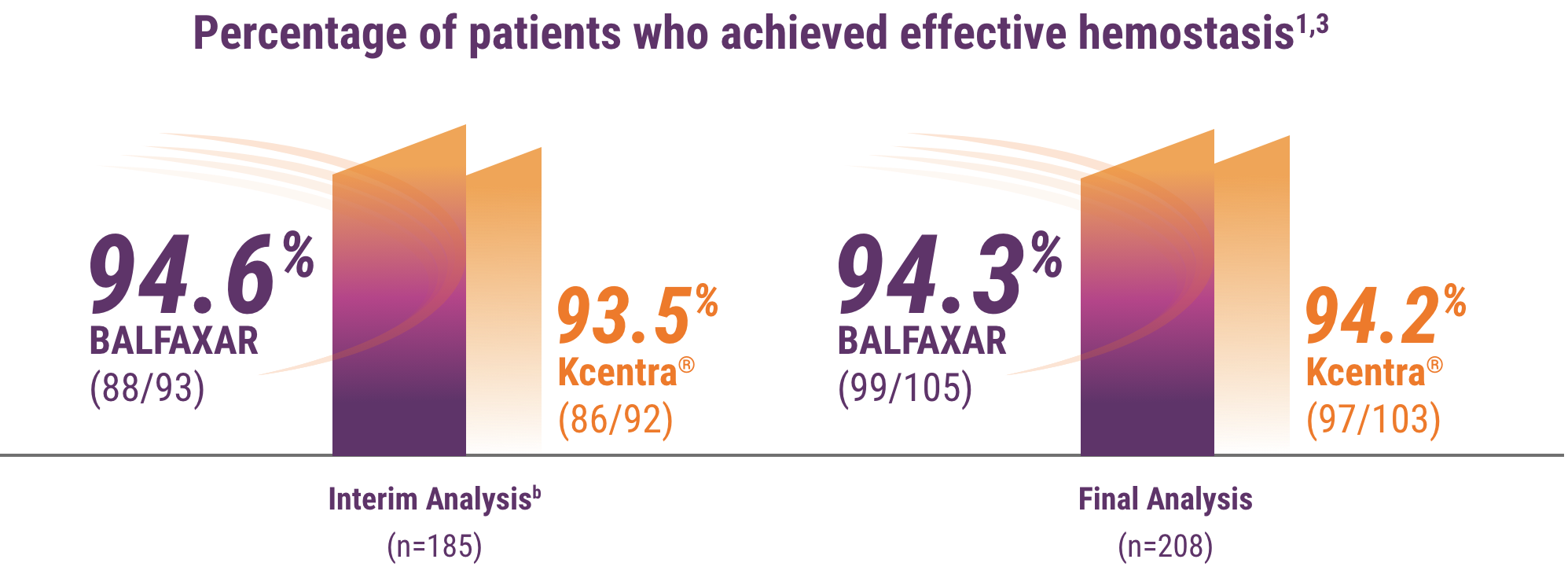
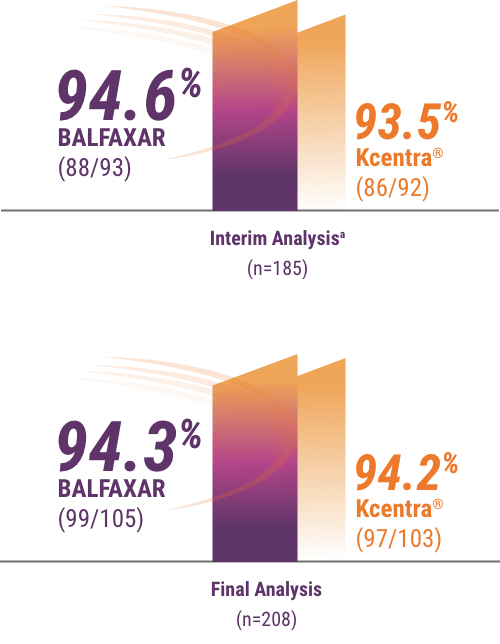
bMet prespecified non-inferiority criterion (margin used -15% and P<0.001, statistically significant at prespecified alpha level of 0.01). Hemostatic efficacy at the end of surgery was assessed by the blinded Independent Endpoint Adjudication Board using objective criteria.
c78.1% in the BALFAXAR group (n=105) vs 71.8% in the Kcentra group (n=103; proportion difference of 6.3%; 95% CI: -5.5%, 18.0%).1
dThe safety and efficacy of repeat dosing of BALFAXAR have not been established.
References: 1. BALFAXAR, Prothrombin Complex Concentrate (Human) Full Prescribing Information. Paramus, NJ: Octapharma USA, Inc. 2. Data on File, Octapharma 2023. 3. Sarode R, Goldstein JN, Simonian G, Milling TJ Jr. A Phase 3, prospective, randomized, double-blind, multicenter, non-inferiority study comparing two four-factor prothrombin complex concentrates for reversal of vitamin K antagonist-induced anticoagulation in patients needing urgent surgery with significant bleeding risk. Blood. 2022;140(Suppl 1):352-353. doi:10.1182/blood-2022-168890
Important Safety InformationView More +
WARNING: ARTERIAL AND VENOUS THROMBOEMBOLIC COMPLICATIONS
Patients being treated with Vitamin K antagonists (VKA) therapy have underlying disease states that predispose them to thromboembolic events. Potential benefits of reversing VKA should be weighed against the potential risks of thromboembolic events, especially in patients with the history of a thromboembolic
Important Safety InformationView Less —
WARNING: ARTERIAL AND VENOUS THROMBOEMBOLIC COMPLICATIONS
Patients being treated with Vitamin K antagonists (VKA) therapy have underlying disease states that predispose them to thromboembolic events. Potential benefits of reversing VKA should be weighed against the potential risks of thromboembolic events, especially in patients with the history of a thromboembolic event. Resumption of anticoagulation should be carefully considered as soon as the risk of thromboembolic events outweighs the risk of acute bleeding. Both fatal and non-fatal arterial and venous thromboembolic complications have been reported with BALFAXAR in clinical trials and post marketing surveillance. Monitor patients receiving BALFAXAR for signs and symptoms of thromboembolic events. BALFAXAR may not be suitable in patients with thromboembolic events in the prior 3 months.
BALFAXAR is contraindicated in patients with known anaphylactic or severe systemic reactions to BALFAXAR or any of its components. BALFAXAR is also contraindicated in patients with a known allergy to heparin, a history of heparin-induced thrombocytopenia (HIT), and IgA deficient patients with known antibodies against IgA.
In clinical trials, the most frequent (≥3%) adverse reactions observed in subjects receiving BALFAXAR were procedural pain, wound complications, asthenia, anemia, dysuria, procedural vomiting, and catheter-site-related reaction.
BALFAXAR is derived from human plasma. The risk of transmission of infectious agents, including viruses and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent and its variant (vCJD), cannot be completely eliminated.
Indications
BALFAXAR (prothrombin complex concentrate, human-lans) is a blood coagulation factor replacement product indicated for the urgent reversal of acquired coagulation factor deficiency induced by Vitamin K antagonist (VKA, e.g., warfarin) therapy in adult patients with need for an urgent surgery/invasive procedure.
Please click here for Full Prescribing Information, including BOXED WARNING.
To report suspected adverse reactions, contact Octapharma USA Inc. at 1-866-766-4860 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.